Page 59 - Chemistry - XI
P. 59
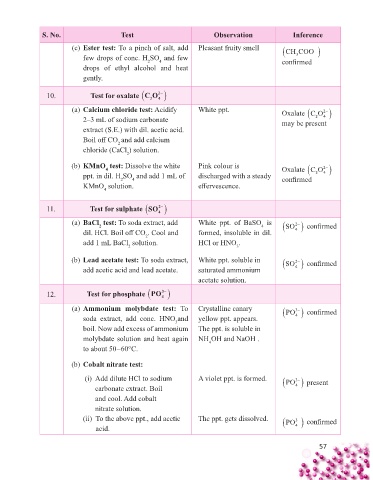
S. No. Test Observation Inference
(c) Ester test: To a pinch of salt, add Pleasant fruity smell CH COO
few drops of conc. H SO and few confi rmed
3
4
2
drops of ethyl alcohol and heat
gently.
2
2
10. Test for oxalate CO
4
(a) Calcium chloride test: Acidify White ppt. Oxalate CO
2
2
2–3 mL of sodium carbonate may be present
4
extract (S.E.) with dil. acetic acid.
Boil off CO and add calcium
2
chloride (CaCl ) solution.
2
(b) KMnO test: Dissolve the white Pink colour is Oxalate CO
2
2
4
ppt. in dil. H SO and add 1 mL of discharged with a steady confi rmed 4
4
2
KMnO solution. eff ervescence.
4
11. Test for sulphate SO
2
4
(a) BaCl test: To soda extract, add White ppt. of BaSO is SO confi rmed
2
4
2
dil. HCl. Boil off CO . Cool and formed, insoluble in dil. 4
2
add 1 mL BaCl solution. HCl or HNO .
2
3
(b) Lead acetate test: To soda extract, White ppt. soluble in SO confi rmed
2
add acetic acid and lead acetate. saturated ammonium 4
acetate solution.
3
12. Test for phosphate PO
4
(a) Ammonium molybdate test: To Crystalline canary PO confi rmed
3
soda extract, add conc. HNO and yellow ppt. appears. 4
3
boil. Now add excess of ammonium The ppt. is soluble in
molybdate solution and heat again NH OH and NaOH .
4
to about 50–60°C.
(b) Cobalt nitrate test:
(i) Add dilute HCl to sodium A violet ppt. is formed. PO present
3
carbonate extract. Boil 4
and cool. Add cobalt
nitrate solution.
(ii) To the above ppt., add acetic The ppt. gets dissolved. PO confi rmed rmed
confi
3
3 3
acid. 4 4 4
57