Page 58 - Chemistry - XI
P. 58
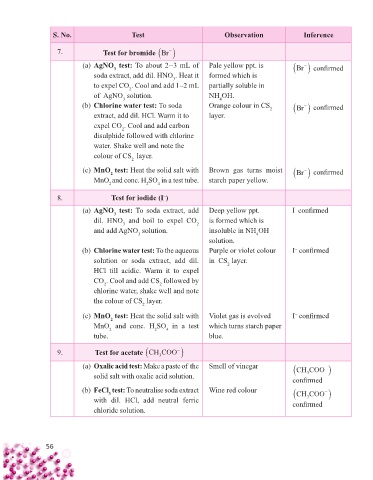
S. No. Test Observation Inference
7. Test for bromide Br
(a) AgNO test: To about 2–3 mL of Pale yellow ppt. is Br confi rmed
3
soda extract, add dil. HNO . Heat it formed which is
3
to expel CO . Cool and add 1–2 mL partially soluble in
2
of AgNO solution. NH OH.
3
4
(b) Chlorine water test: To soda Orange colour in CS 2 Br confi rmed
extract, add dil. HCl. Warm it to layer.
expel CO . Cool and add carbon
2
disulphide followed with chlorine
water. Shake well and note the
colour of CS layer.
2
(c) MnO test: Heat the solid salt with Brown gas turns moist Br confi rmed
2
MnO and conc. H SO in a test tube. starch paper yellow.
2 2 4
8. Test for iodide (I )
–
(a) AgNO test: To soda extract, add Deep yellow ppt. I confi rmed
–
3
dil. HNO and boil to expel CO 2 is formed which is
3
and add AgNO solution. insoluble in NH OH
3 4
solution.
(b) Chlorine water test: To the aqueous Purple or violet colour I confi rmed
–
solution or soda extract, add dil. in CS layer.
2
HCl till acidic. Warm it to expel
CO . Cool and add CS followed by
2
2
chlorine water, shake well and note
the colour of CS layer.
2
(c) MnO test: Heat the solid salt with Violet gas is evolved I confi rmed
–
2
MnO and conc. H SO in a test which turns starch paper
2
4
2
tube. blue.
9. Test for acetate CH COO
3
(a) Oxalic acid test: Make a paste of the Smell of vinegar CH COO
solid salt with oxalic acid solution. 3
confi rmed
(b) FeCl test: To neutralise soda extract Wine red colour CH COO
3
with dil. HCl, add neutral ferric confi rmed
3
chloride solution.
56