Page 57 - Chemistry - XI
P. 57
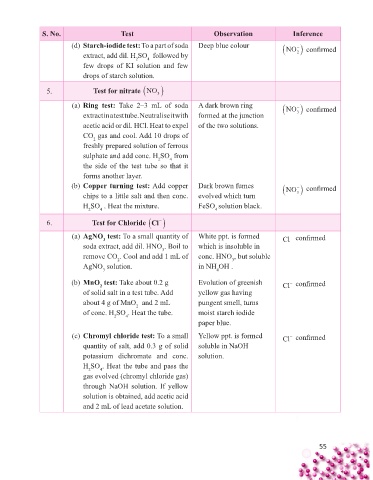
S. No. Test Observation Inference
(d) Starch-iodide test: To a part of soda Deep blue colour NO confi rmed
extract, add dil. H SO followed by 2
4
2
few drops of KI solution and few
drops of starch solution.
5. Test for nitrate NO
3
(a) Ring test: Take 2–3 mL of soda A dark brown ring NO confi rmed
extract in a test tube. Neutralise it with formed at the junction 3
acetic acid or dil. HCl. Heat to expel of the two solutions.
CO gas and cool. Add 10 drops of
2
freshly prepared solution of ferrous
sulphate and add conc. H SO from
2
4
the side of the test tube so that it
forms another layer.
(b) Copper turning test: Add copper Dark brown fumes NO confi rmed
chips to a little salt and then conc. evolved which turn 3
H SO . Heat the mixture. FeSO solution black.
2 4 4
6. Test for Chloride Cl
(a) AgNO test: To a small quantity of White ppt. is formed Cl − confi rmed
3
soda extract, add dil. HNO . Boil to which is insoluble in
3
remove CO . Cool and add 1 mL of conc. HNO , but soluble
3
2
AgNO solution. in NH OH .
4
3
(b) MnO test: Take about 0.2 g Evolution of greenish Cl − confi rmed
2
of solid salt in a test tube. Add yellow gas having
about 4 g of MnO and 2 mL pungent smell, turns
2
of conc. H SO . Heat the tube. moist starch iodide
4
2
paper blue.
(c) Chromyl chloride test: To a small Yellow ppt. is formed Cl − confi rmed
quantity of salt, add 0.3 g of solid soluble in NaOH
potassium dichromate and conc. solution.
H SO . Heat the tube and pass the
4
2
gas evolved (chromyl chloride gas)
through NaOH solution. If yellow
solution is obtained, add acetic acid
and 2 mL of lead acetate solution.
55