Page 56 - Chemistry - XI
P. 56
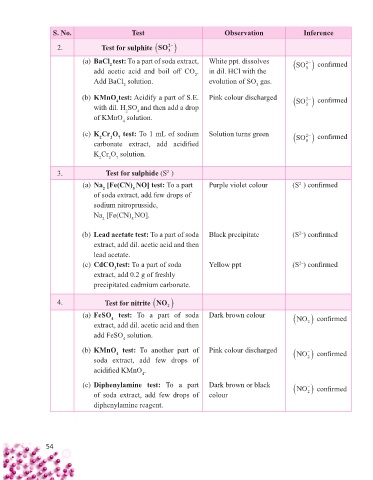
S. No. Test Observation Inference
2
2. Test for sulphite SO
3
(a) BaCl test: To a part of soda extract, White ppt. dissolves SO confi rmed
2
2
add acetic acid and boil off CO . in dil. HCl with the 3
2
Add BaCl solution. evolution of SO gas.
2 2
(b) KMnO test: Acidify a part of S.E. Pink colour discharged SO confi rmed
2
4
with dil. H SO and then add a drop 3
4
2
of KMnO solution.
4
(c) K Cr O test: To 1 mL of sodium Solution turns green SO confi rmed
2
2
2
7
carbonate extract, add acidifi ed 3
K Cr O solution.
2
7
2
3. Test for sulphide (S )
2–
(a) Na [Fe(CN) NO] test: To a part Purple violet colour (S ) confi rmed
2–
2
5
of soda extract, add few drops of
sodium nitroprusside,
Na [Fe(CN) NO].
2 5
(b) Lead acetate test: To a part of soda Black precipitate (S ) confi rmed
2–
extract, add dil. acetic acid and then
lead acetate.
(c) CdCO test: To a part of soda Yellow ppt (S ) confi rmed
2–
3
extract, add 0.2 g of freshly
precipitated cadmium carbonate.
4. Test for nitrite NO
2
(a) FeSO test: To a part of soda Dark brown colour NO confi rmed
4
extract, add dil. acetic acid and then 2
add FeSO solution.
4
(b) KMnO test: To another part of Pink colour discharged NO confi rmed
4
soda extract, add few drops of 2
acidifi ed KMnO .
4
(c) Diphenylamine test: To a part Dark brown or black NO confi rmed
of soda extract, add few drops of colour 2
diphenylamine reagent.
54