Page 64 - Chemistry - XI
P. 64
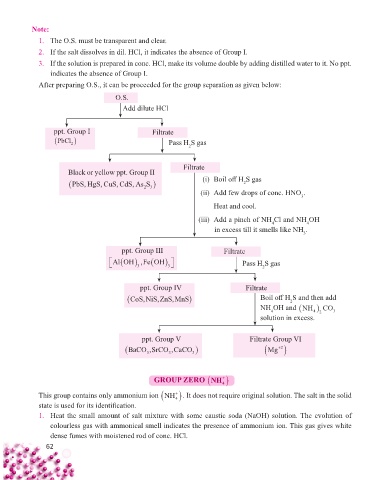
Note:
1. The O.S. must be transparent and clear.
2. If the salt dissolves in dil. HCl, it indicates the absence of Group I.
3. If the solution is prepared in conc. HCl, make its volume double by adding distilled water to it. No ppt.
indicates the absence of Group I.
After preparing O.S., it can be proceeded for the group separation as given below:
O.S.
Add dilute HCl
ppt. Group I Filtrate
PbCl Pass H S gas
2
2
Filtrate
Black or yellow ppt. Group II
PbS, HgS, CuS, CdS, As S (i) Boil off H S gas
2
23
(ii) Add few drops of conc. HNO .
3
Heat and cool.
(iii) Add a pinch of NH Cl and NH OH
4
4
in excess till it smells like NH .
3
ppt. Group III Filtrate
Al OH ,FeOH Pass H S gas
3
2
3
ppt. Group IV Filtrate
CoS,NiS,ZnS,MnS Boil off H S and then add
2
NH OH and NH CO 3
4
solution in excess. 4 2
ppt. Group V Filtrate Group VI
BaCO ,SrCO,CaCO Mg
2
3
3
3
GROUP ZERO NH
+
4
This group contains only ammonium ion NH . It does not require original solution. The salt in the solid
4
state is used for its identifi cation.
1. Heat the small amount of salt mixture with some caustic soda (NaOH) solution. The evolution of 1. Heat the small amount of salt mixture with some caustic soda (NaOH) solution. The evolution of
colourless gas with ammonical smell indicates the presence of ammonium ion. This gas gives white colourless gas with ammonical smell indicates the presence of ammonium ion. This gas gives white
dense fumes with moistened rod of conc. HCl.dense fumes with moistened rod of conc. HCl.
62