Page 66 - Chemistry - XI
P. 66
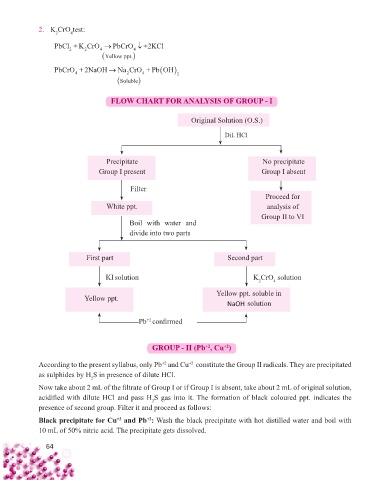
2. K CrO test:
4
2
PbCl +K CrO PbCrO +2KCl
2 2 4 4
Yellow pppt.
PbCrO+ 2NaOH NaCrO +PbOH
4 2 4 2
S oluble
FLOW CHART FOR ANALYSIS OF GROUP - I
Original Solution (O.S.)
Dil. HCl
Precipitate No precipitate
Group I present Group I absent
Filter
Proceed for
White ppt. analysis of
Group II to VI
Boil with water and
divide into two parts
First part Second part
KI solution K CrO solution
2 4
Yellow ppt. soluble in
Yellow ppt.
NaOH solution
Pb confi rmed
+2
GROUP - II (Pb , Cu )
+2
+2
According to the present syllabus, only Pb and Cu constitute the Group II radicals. They are precipitated
+2
+2
as sulphides by H S in presence of dilute HCl.
2
Now take about 2 mL of the fi ltrate of Group I or if Group I is absent, take about 2 mL of original solution,
acidifi ed with dilute HCl and pass H S gas into it. The formation of black coloured ppt. indicates the
2
presence of second group. Filter it and proceed as follows:
Black precipitate for Cu and Pb : Wash the black precipitate with hot distilled water and boil withBlack precipitate for Cu +2 +2
10 mL of 50% nitric acid. The precipitate gets dissolved.10 mL of 50% nitric acid. The precipitate gets dissolved.
64