Page 71 - Chemistry - XI
P. 71
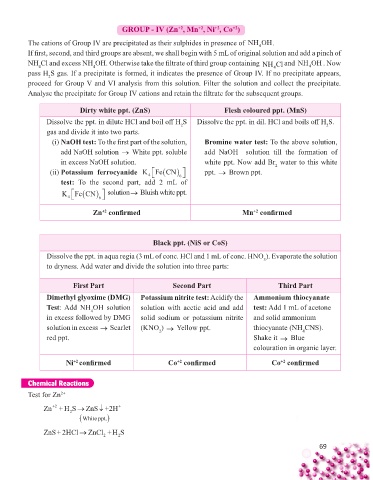
GROUP - IV (Zn , Mn , Ni , Co )
+2
+2
+2
+2
The cations of Group IV are precipitated as their sulphides in presence of NH OH.
4
If fi rst, second, and third groups are absent, we shall begin with 5 mL of original solution and add a pinch of
NH Cl and excess NH OH. Otherwise take the fi ltrate of third group containing NH Cl and NH OH . Now
4
4
4
4
pass H S gas. If a precipitate is formed, it indicates the presence of Group IV. If no precipitate appears,
2
proceed for Group V and VI analysis from this solution. Filter the solution and collect the precipitate.
Analyse the precipitate for Group IV cations and retain the fi ltrate for the subsequent groups.
Dirty white ppt. (ZnS) Flesh coloured ppt. (MnS)
Dissolve the ppt. in dilute HCl and boil off H S Dissolve the ppt. in dil. HCl and boils off H S.
2
2
gas and divide it into two parts.
(i) NaOH test: To the fi rst part of the solution, Bromine water test: To the above solution,
add NaOH solution → White ppt. soluble add NaOH solution till the formation of
in excess NaOH solution. white ppt. Now add Br water to this white
2
(ii) Potassium ferrocyanide KFeCN ppt. → Brown ppt.
4
6
test: To the second part, add 2 mL of
KFeCN solution→ Bluish white ppt.
4
6
Zn confi rmed Mn confi rmed
+2
+2
Black ppt. (NiS or CoS)
Dissolve the ppt. in aqua regia (3 mL of conc. HCl and 1 mL of conc. HNO ). Evaporate the solution
3
to dryness. Add water and divide the solution into three parts:
First Part Second Part Third Part
Dimethyl glyoxime (DMG) Potassium nitrite test: Acidify the Ammonium thiocyanate
Test: Add NH OH solution solution with acetic acid and add test: Add 1 mL of acetone
4
in excess followed by DMG solid sodium or potassium nitrite and solid ammonium
solution in excess → Scarlet (KNO ) → Yellow ppt. thiocyanate (NH CNS).
4
2
red ppt. Shake it → Blue
colouration in organic layer.
Ni confi rmed Co confi rmed Co confi rmed
+2
+2
+2
Chemical Reactions
Test for Zn 2+
Zn +H S ZnS +2H +
+2
2
White ppt.
ZnS+ 2HCl → ZnCl +H S
2 2
69