Page 76 - Chemistry - XI
P. 76
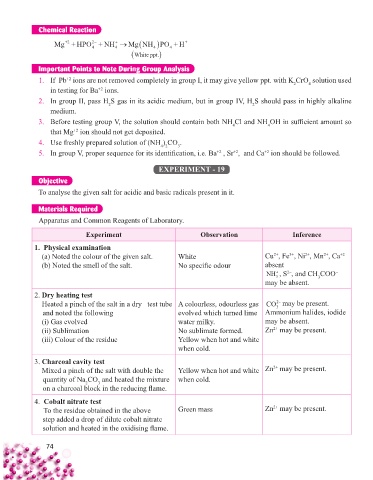
Chemical Reaction
Mg +HPO +NH MgNHPO+ H +
2
+2
4 4 4 4
White ppt.
Important Points to Note During Group Analysis
1. If Pb ions are not removed completely in group I, it may give yellow ppt. with K CrO solution used
+2
2
4
in testing for Ba ions.
+2
2. In group II, pass H S gas in its acidic medium, but in group IV, H S should pass in highly alkaline
2
2
medium.
3. Before testing group V, the solution should contain both NH Cl and NH OH in suffi cient amount so
4
4
that Mg ion should not get deposited.
+2
4. Use freshly prepared solution of (NH ) CO .
4 2
3
5. In group V, proper sequence for its identifi cation, i.e. Ba , Sr , and Ca ion should be followed.
+2
+2
+2
EXPERIMENT - 19
Objective
To analyse the given salt for acidic and basic radicals present in it.
Materials Required
Apparatus and Common Reagents of Laboratory.
Experiment Observation Inference
1. Physical examination
(a) Noted the colour of the given salt. White Cu , Fe , Ni , Mn , Ca +2
2+
2+
2+
3+
(b) Noted the smell of the salt. No specifi c odour absent
NH , S , and CH COO –
2–
+
3
3
may be absent.
2. Dry heating test
Heated a pinch of the salt in a dry test tube A colourless, odourless gas CO may be present.
2−
3
and noted the following evolved which turned lime Ammonium halides, iodide
(i) Gas evolved water milky. may be absent.
2+
(ii) Sublimation No sublimate formed. Zn may be present.
(iii) Colour of the residue Yellow when hot and white
when cold.
3. Charcoal cavity test
2+
Mixed a pinch of the salt with double the Yellow when hot and white Zn may be present.
quantity of Na CO and heated the mixture when cold.
3
2
on a charcoal block in the reducing fl ame.
4. Cobalt nitrate test
2+
To the residue obtained in the above Green mass Zn may be present.
step added a drop of dilute cobalt nitrate
step added a drop of dilute cobalt nitrate
solution and heated in the oxidising fl ame.
solution and heated in the oxidising fl ame.
74