Page 75 - Chemistry - XI
P. 75
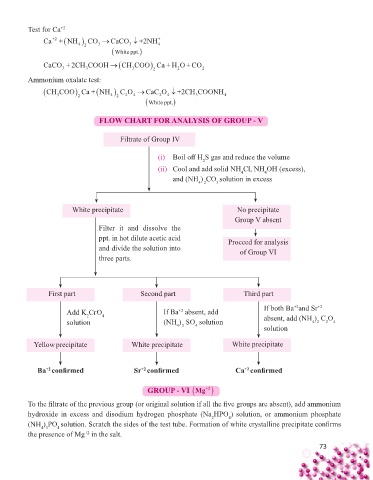
Test for Ca +2
Ca +NH CO CaCO +2NH +
+2
4 2 3 3 4
White ppt.
CaCO +2CH COOH CH COOCa+ HO +CO
3 3 3 2 2 2
Ammonium oxalate test:
CH COOCa+ NH CO CaCO +2CH COONH 4
3
4
2
2
3
4
4 2
2
White ppt.
FLOW CHART FOR ANALYSIS OF GROUP - V
Filtrate of Group IV
(i) Boil off H S gas and reduce the volume
2
(ii) Cool and add solid NH Cl, NH OH (excess),
4
4
and (NH ) CO solution in excess
4 2 3
White precipitate No precipitate
Group V absent
Filter it and dissolve the
ppt. in hot dilute acetic acid Proceed for analysis
and divide the solution into of Group VI
three parts.
First part Second part Third part
+2
Add K CrO 4 If Ba absent, add If both Ba and Sr +2
+2
2
solution (NH ) SO solution absent, add (NH ) C O 4
4 2
2
4 2
4
solution
Yellow precipitate White precipitate White precipitate
Ba confi rmed Sr confi rmed Ca confi rmed
+2
+2
+2
GROUP - VI Mg
+2
To the fi ltrate of the previous group (or original solution if all the fi ve groups are absent), add ammonium
hydroxide in excess and disodium hydrogen phosphate (Na HPO ) solution, or ammonium phosphate ) solution, or ammonium phosphate
2
4
(NH ) PO solution. Scratch the sides of the test tube. Formation of white crystalline precipitate confi rms solution. Scratch the sides of the test tube. Formation of white crystalline precipitate confi rms
4 3
4
the presence of Mg in the salt.
+2
73