Page 77 - Chemistry - XI
P. 77
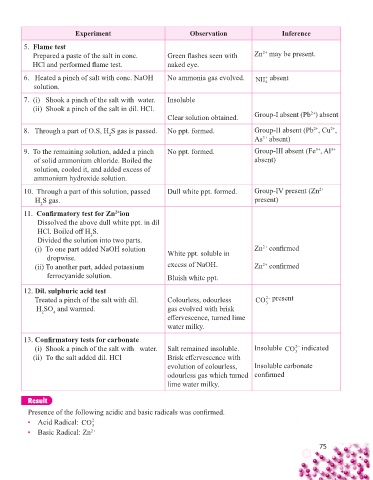
Experiment Observation Inference
5. Flame test
2+
Prepared a paste of the salt in conc. Green fl ashes seen with Zn may be present.
HCl and performed fl ame test. naked eye.
6. Heated a pinch of salt with conc. NaOH No ammonia gas evolved. NH + absent
solution. 4
7. (i) Shook a pinch of the salt with water. Insoluble
(ii) Shook a pinch of the salt in dil. HCl.
2+
Clear solution obtained. Group-I absent (Pb ) absent
2+
2+
8. Through a part of O.S, H S gas is passed. No ppt. formed. Group-II absent (Pb , Cu ,
2
As absent)
3+
9. To the remaining solution, added a pinch No ppt. formed. Group-III absent (Fe , Al 3+
3+
of solid ammonium chloride. Boiled the absent)
solution, cooled it, and added excess of
ammonium hydroxide solution.
10. Through a part of this solution, passed Dull white ppt. formed. Group-IV present (Zn 2+
H S gas. present)
2
11. Confi rmatory test for Zn ion
2+
Dissolved the above dull white ppt. in dil
HCl. Boiled off H S.
2
Divided the solution into two parts.
(i) To one part added NaOH solution Zn confi rmed
2+
dropwise. White ppt. soluble in
(ii) To another part, added potassium excess of NaOH. Zn confi rmed
2+
ferrocyanide solution. Bluish white ppt.
12. Dil. sulphuric acid test
Treated a pinch of the salt with dil. Colourless, odourless CO 2− present
H SO and warmed. gas evolved with brisk 3
2 4
eff ervescence, turned lime
water milky.
13. Confi rmatory tests for carbonate
(i) Shook a pinch of the salt with water. Salt remained insoluble. Insoluble CO indicated
2−
(ii) To the salt added dil. HCl Brisk eff ervescence with 3
evolution of colourless, Insoluble carbonate
odourless gas which turned confi rmed
lime water milky.
Result
Presence of the following acidic and basic radicals was confi rmed.
2−
• Acid Radical: CO
3
• Basic Radical: Zn 2+
75