Page 70 - Chemistry - XI
P. 70
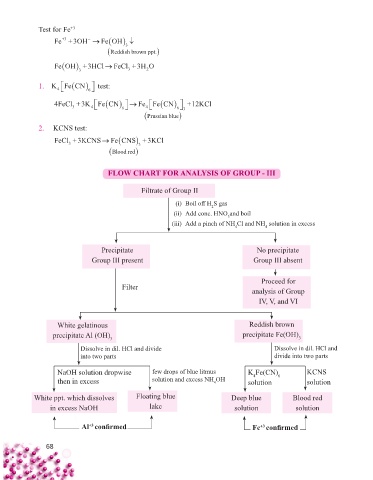
Test for Fe +3
Fe +3OH
Fe OH
+3
3
Reddish brown ppt.
Fe OH +3HCl FeCl+3H O
3 3 2
1. KFeCN test:
6
4
4FeCl+3K Fe CN Fe Fe CN +12KCl
3 4 6 4 6 3
Prussian blue
2. KCNS test:
FeCl +3KCNS
Fe CNS+3KCl
3 3
Blood red
FLOW CHART FOR ANALYSIS OF GROUP - III
Filtrate of Group II
(i) Boil off H S gas
2
(ii) Add conc. HNO and boil
3
(iii) Add a pinch of NH Cl and NH solution in excess
4
4
Precipitate No precipitate
Group III present Group III absent
Proceed for
Filter
analysis of Group
IV, V, and VI
White gelatinous Reddish brown
precipitate Al (OH) 3 precipitate Fe(OH) 3
Dissolve in dil. HCl and divide Dissolve in dil. HCl and
into two parts divide into two parts
NaOH solution dropwise few drops of blue litmus K Fe(CN) KCNS
4
then in excess solution and excess NH OH solution 6 solution
4
White ppt. which dissolves Floating blue Deep blue Blood red
in excess NaOH lake solution solution
Al confi rmed Fe confi rmed
+3
+3
68