Page 113 - Chemistry - XI
P. 113
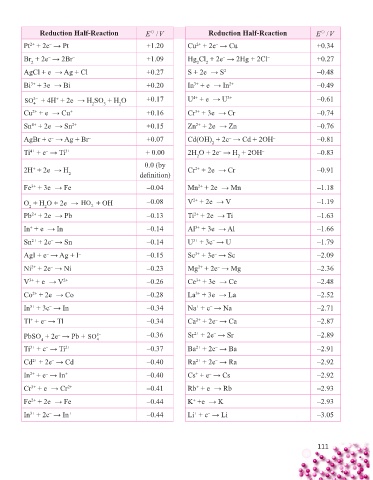
Reduction Half-Reaction E / V Reduction Half-Reaction E / V
Pt + 2e → Pt +1.20 Cu + 2e → Cu +0.34
–
2+
–
2+
Br + 2e → 2Br – +1.09 Hg Cl + 2e → 2Hg + 2Cl – +0.27
–
–
2 2 2
AgCl + e → Ag + Cl – +0.27 S + 2e → S 2– –0.48
–
–
Bi + 3e → Bi +0.20 In + e → In 2+ –0.49
3+
–
3+
–
4+
–
SO + 4H + 2e → H SO + H O +0.17 U + e → U 3+ –0.61
2−
–
+
2
3
2
4
Cu + e → Cu + +0.16 Cr + 3e → Cr –0.74
3+
2+
–
–
Sn + 2e → Sn 2+ +0.15 Zn + 2e → Zn –0.76
4+
–
–
2+
AgBr + e → Ag + Br – +0.07 Cd(OH) + 2e → Cd + 2OH – –0.81
–
–
2
Ti + e → Ti 3+ + 0.00 2H O + 2e → H + 2OH – –0.83
–
–
4+
2 2
0.0 (by
2H + 2e → H Cr + 2e → Cr –0.91
2+
–
–
+
2 defi nition)
Fe + 3e → Fe –0.04 Mn + 2e → Mn –1.18
3+
2+
–
–
–
2+
−
O + H O + 2e → HO + OH – –0.08 V + 2e → V –1.19
–
2
2
2
Pb + 2e → Pb –0.13 Ti + 2e → Ti –1.63
–
–
2+
2+
In + e → In –0.14 Al + 3e → Al –1.66
3+
–
–
+
Sn + 2e → Sn –0.14 U + 3e → U –1.79
–
3+
2+
–
AgI + e → Ag + I – –0.15 Sc + 3e → Sc –2.09
–
3+
–
Ni + 2e → Ni –0.23 Mg + 2e → Mg –2.36
–
–
2+
2+
V + e → V 2+ –0.26 Ce + 3e → Ce –2.48
3+
–
3+
–
Co + 2e → Co –0.28 La + 3e → La –2.52
–
3+
2+
–
In + 3e → In –0.34 Na + e → Na –2.71
–
–
+
3+
Tl + e → Tl –0.34 Ca + 2e → Ca –2.87
2+
–
+
–
2+
–
– 2− –0.36 Sr + 2e → Sr –2.89
PbSO + 2e → Pb + SO
4 4
Ti + e → Ti 2+ –0.37 Ba + 2e → Ba –2.91
2+
–
–
3+
Cd + 2e → Cd –0.40 Ra + 2e → Ra –2.92
–
2+
2+
–
In + e → In + –0.40 Cs + e → Cs –2.92
+
–
2+
–
Cr + e → Cr 2+ –0.41 Rb + e → Rb –2.93
3+
–
–
+
Fe + 2e → Fe –0.44 K +e → K –2.93
2+
–
–
+
In + 2e → In + –0.44 Li + e → Li –3.05
–3.05
–
–
+
3+
111