Page 112 - Chemistry - XI
P. 112
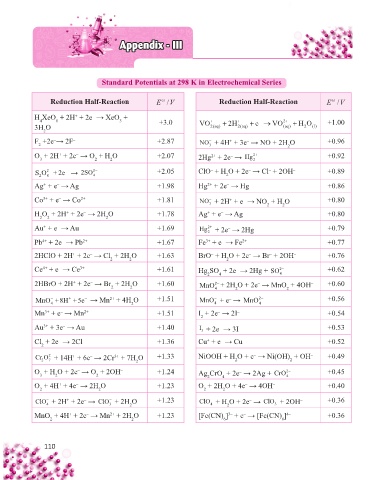
Appendix - III
Appendix - III
Standard Potentials at 298 K in Electrochemical Series
Reduction Half-Reaction E / V Reduction Half-Reaction E / V
H XeO + 2H + 2e → XeO + 2
–
+
6
3
4
3H O +3.0 VO 2( aq) 2 H 2( aq) e VO ( aq) HO l () +1.00
2
2
F +2e → 2F – +2.87 NO + 4H + 3e → NO + 2H O +0.96
–
−
–
+
2 3 2
O + 2H + 2e → O + H O +2.07 2+ – 2+ +0.92
–
+
3 2 2 2Hg + 2e → Hg 2
–
–
–
2− − 2− +2.05 ClO + H O + 2e → Cl + 2OH – +0.89
SO +2e → 2SO
2 8 4 2
Ag + e → Ag +1.98 Hg + 2e → Hg +0.86
+
–
–
2+
Co + e → Co 2+ +1.81 NO + 2H + e → NO + H O +0.80
–
3+
−
+
–
3 2 2
H O + 2H + 2e → 2H O +1.78 Ag + e → Ag +0.80
+
–
–
+
2 2 2
Au + e → Au +1.69 Hg + 2e → 2Hg +0.79
+
–
–
2+
2
Pb + 2e → Pb 2+ +1.67 Fe + e → Fe 2+ +0.77
–
–
4+
3+
2HClO + 2H + 2e → Cl + 2H O +1.63 BrO + H O + 2e → Br + 2OH – +0.76
+
–
–
–
–
2 2 2
Ce + e → Ce 3+ +1.61 Hg SO + 2e → 2Hg + SO 2− +0.62
–
4+
–
4
2
4
2HBrO + 2H + 2e → Br + 2H O +1.60 MnO + 2H O + 2e → MnO + 4OH – +0.60
–
+
2−
–
2
2
2
2
4
MnO+8H +5e → Mn + 4H O +1.51 MnO + e → MnO 2− +0.56
−
−
−
–
2+
+
4 2 4 4
Mn + e → Mn 2+ +1.51 I + 2e → 2I – +0.54
–
–
3+
2
Au + 3e → Au +1.40 I 3 + 2e → 3I – +0.53
–
3+
–
Cl + 2e → 2Cl – +1.36 Cu + e → Cu +0.52
–
+
–
2
–
Cr O + 14H + 6e → 2Cr + 7H O +1.33 NiOOH + H O + e → Ni(OH) + OH – +0.49
2−
+
3+
–
2
2
2
7
2
O + H O + 2e → O + 2OH – +1.24 – 2− +0.45
–
3 2 2 Ag CrO + 2e → 2Ag + CrO 4
4
2
O + 4H + 4e → 2H O +1.23 O + 2H O + 4e → 4OH – +0.40
–
+
–
2 2 2 2
ClO + 2H + 2e → ClO + 2H O +1.23 ClO + H O + 2e → ClO + 2OH – +0.36
−
−
–
–
+
−
−
4 3 2 4 2 3
MnO
MnO + 4H + 2e → Mn + 2H O + 4H + 2e – 2+ +1.23 [Fe(CN) ] + e → [Fe(CN) ] 4– +0.36
3–
–
+
2 2 2 6 6
110
110