Page 116 - Chemistry - XI
P. 116
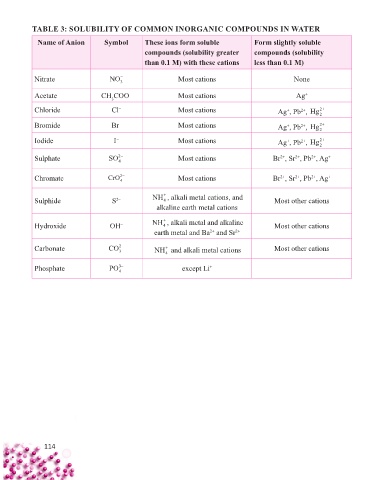
TABLE 3: SOLUBILITY OF COMMON INORGANIC COMPOUNDS IN WATER
Name of Anion Symbol These ions form soluble Form slightly soluble
compounds (solubility greater compounds (solubility
than 0.1 M) with these cations less than 0.1 M)
Nitrate NO − 3 Most cations None
Acetate CH COO – Most cations Ag +
3
Chloride Cl – Most cations Ag , Pb , Hg 2+
2+
+
2
Bromide Br – Most cations Ag , Pb , Hg 2+
+
2+
2
Iodide I – Most cations + 2+ 2+
Ag , Pb , Hg
2
Sulphate SO 2− Most cations Br , Sr , Pb , Ag +
2+
2+
2+
4
Chromate CrO 2− Most cations Br , Sr , Pb , Ag +
2+
2+
2+
4
+
Sulphide S 2– NH , alkali metal cations, and Most other cations
4
alkaline earth metal cations
+
Hydroxide OH – NH , alkali metal and alkaline Most other cations
4
earth metal and Ba and Sr 2+
2+
Carbonate CO 2− NH and alkali metal cations Most other cations
+
3
4
Phosphate PO 3− except Li +
4
114