Page 42 - Chemistry - XI
P. 42
5. Calculating percentage purity of a sample:
% purity Calculated strength 100
Givenstrength
6. Conversion of normality into molarity:
N = n × M
Molecularmass
n =
Equivalent mass
Acid-Base Titrations (Acidimetry and Alkalimetry)
In these titrations, the two solutions to be titrated against each other are an acid and a base. From these
titrations we can determine the strength of an acid or the strength of a base by titration against each other.
They are also known as acidimetry and alkalimetry, respectively.
For titrating a weak acid like acetic acid or oxalic acid, we use a strong base like NaOH; and for titrating
a weak base like sodium carbonate or ammonia, we use a strong acid like HCl, H SO , etc. For titrating a
4
2
strong base or a strong acid, we can use a strong or weak acid or base.
Indicators used in acidimetry and alkalimetry: Acid base indicators are sensitive to pH change. The
important indicators commonly used in acid-alkali titrations are phenolphthalein (pink in alkali and
colourless in acid) and methyl orange (yellow in alkali and red in acid).
The choice of an indicator depends upon the nature of the acid and the alkali employed in the titration.
Acid (in the burette) Alkali (in the titration fl ash) Indicator End point
Strong acid Strong alkali 1. Phenolphthalein Pink to colourless
(HCl, HNO ,H SO ) (NaOH, KOH) 2. Methyl orange Yellow to pink
3 2 4
Weak acid (Oxalic acid, acetic acid) Strong alkali (NaOH, KOH) Phenolphthalein Pink to colourless
Weak alkali (Na CO , K CO ,
Strong acid (HCl, HNO , H SO ) 2 3 2 3 Methyl orange Yellow to pink
3 2 4 NaHCO , KHCO )
3 3
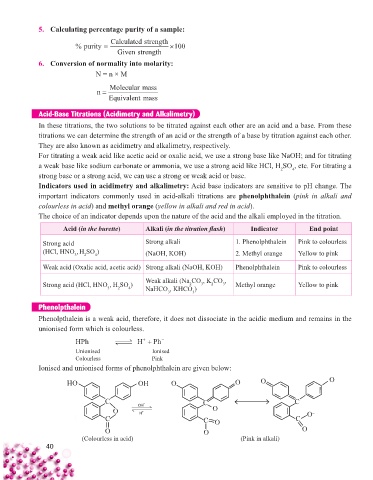
Phenolpthalein
Phenolpthalein is a weak acid, therefore, it does not dissociate in the acidic medium and remains in the
unionised form which is colourless.
HPh H Ph
Unionised Ionised
Colourless Pink
Ionised and unionised forms of phenolphthalein are given below:
OH OH O – O O O –
C C C
OH
O O – O –
H
C C O C
O O – O
(Colourless in acid) (Pink in alkali)
40