Page 38 - Chemistry - XI
P. 38
9. Standard solution: A solution whose strength is known is called a standard solution.
10. Equivalent weight of a substance is defi ned as the number of parts by weight of it that combines with
or displaces directly or indirectly one part by weight of hydrogen or eight parts by weight of oxygen or
35.5 parts by weight of chlorine.
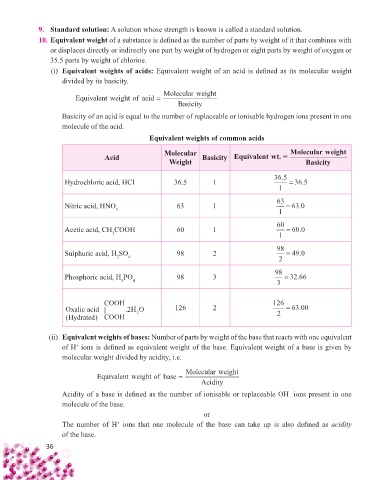
(i) Equivalent weights of acids: Equivalent weight of an acid is defi ned as its molecular weight
divided by its basicity.
Molecularweight
Equivalent weight of acid =
Basicity
Basicity of an acid is equal to the number of replaceable or ionisable hydrogen ions present in one
molecule of the acid.
Equivalent weights of common acids
Molecular Molecularweight
Acid Basicity Equivalentwt. =
Weight Basicity
Hydrochloric acid, HCl 36.5 1 36 5. = 36 5.
1
Nitric acid, HNO 63 1 63 = 63 0.
3
1
Acetic acid, CH COOH 60 1 60 = 60 0.
3
1
Sulphuric acid, H SO 98 2 98 = 49 0.
2
4
2
Phosphoric acid, H PO 98 3 98 = 32 66.
4
3
3
COOH 126 2 126
Oxalic acid | .2 HO = 63 00.
2 2
( Hydrated) COOH
(ii) Equivalent weights of bases: Number of parts by weight of the base that reacts with one equivalent
of H ions is defi ned as equivalent weight of the base. Equivalent weight of a base is given by
+
molecular weight divided by acidity, i.e.
Equivalent weight of base = Molecularweight
Acidity
Acidity of a base is defi ned as the number of ionisable or replaceable OH ions present in one
–
molecule of the base.
or
or
The number of H ions that one molecule of the base can take up is also defi ned as acidityThe number of H +
of the base.
of the base.
36