Page 40 - Chemistry - XI
P. 40
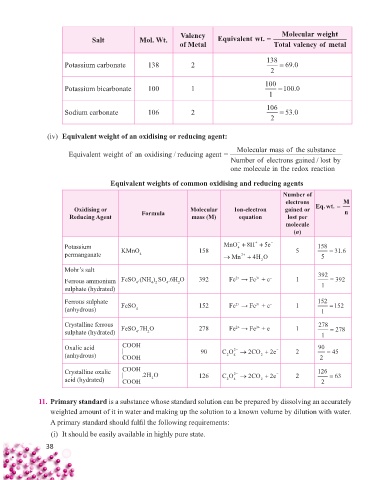
Valency Molecularweight
Salt Mol. Wt. Equivalentwt. =
of Metal Total valency of metal
Potassium carbonate 138 2 138 = 69 0.
2
Potassium bicarbonate 100 1 100 = 100 0.
1
Sodium carbonate 106 2 106 = 53 0.
2
(iv) Equivalent weight of an oxidising or reducing agent:
Molecularmassoffthe substance
Equivalent weight of an oxidising /reducingagent =
Number of electrons gained /lostby
one molecule in thhe redox reaction
Equivalent weights of common oxidising and reducing agents
Number of
electrons M
Oxidising or Molecular Ion-electron gained or Eq.wt. = n
Reducing Agent Formula mass (M) equation lost per
molecule
(n)
Potassium KMnO 158 MnO 8 H e 5 5 158
4
permanganate 4 2 5 = 31 6.
Mn
4
HO
2
Mohr’s salt
3+
2+
Ferrous ammonium FeSO .(NH ) SO .6H O 392 Fe → Fe + e – 1 392 = 392
2
4 2
4
4
sulphate (hydrated) 1
Ferrous sulphate 2+ 3+ – 152
(anhydrous) FeSO 4 152 Fe → Fe + e 1 1 = 152
Crystalline ferrous FeSO .7H O 278 Fe → Fe + e – 1 278
3+
2+
sulphate (hydrated) 4 2 = 278
1
Oxalic acid COOH 2 90
(anhydrous) | COOH 90 CO 4 2 CO e 2 2 2 = 45
2
2
Crystalline oxalic COOH 126 2 2 126
acid (hydrated) | COOH .2 HO CO 4 2 CO e 2 2 = 63
2
2
2
11. Primary standard is a substance whose standard solution can be prepared by dissolving an accurately
weighted amount of it in water and making up the solution to a known volume by dilution with water.
weighted amount of it in water and making up the solution to a known volume by dilution with water.
A primary standard should fulfi l the following requirements:
A primary standard should fulfi l the following requirements:
(i) It should be easily available in highly pure state.
(i) It should be easily available in highly pure state.
38