Page 39 - Chemistry - XI
P. 39
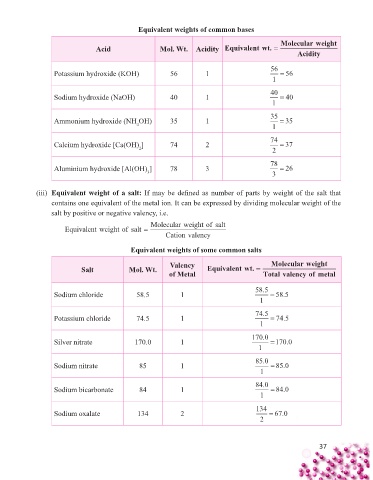
Equivalent weights of common bases
Molecularweight
Acid Mol. Wt. Acidity Equivalentwt. =
Acidity
Potassium hydroxide (KOH) 56 1 56 = 56
1
Sodium hydroxide (NaOH) 40 1 40 = 40
1
Ammonium hydroxide (NH OH) 35 1 35 = 35
4
1
Calcium hydroxide [Ca(OH) ] 74 2 74 = 37
2
2
Aluminium hydroxide [Al(OH) ] 78 3 78 = 26
3
3
(iii) Equivalent weight of a salt: If may be defi ned as number of parts by weight of the salt that
contains one equivalent of the metal ion. It can be expressed by dividing molecular weight of the
salt by positive or negative valency, i.e.
Equivalent weight of salt = Molecularweight of salt
Cationvalency
Equivalent weights of some common salts
Valency Molecularweight
Salt Mol. Wt. Equivalentwt. =
of Metal Total valency of metal
Sodium chloride 58.5 1 58 5. = 58 5.
1
Potassium chloride 74.5 1 74 5. = 74 5.
1
Silver nitrate 170.0 1 170 0. = 170 0.
1
Sodium nitrate 85 1 85 0. = 85 0.
1
Sodium bicarbonate 84 1 84 0. = 84 0.
1
Sodium oxalate 134 2 134 = 67 0.
2
37