Page 104 - Chemistry - XI
P. 104
(ii) Translucent spot test: Rub the piece of oil on a fi lter paper. A translucent spot appears in the
fi lter paper.
(iii) Acrolein test: A small amount of fat is heated strongly with potassium bisulphate crystals in a dry
test tube. A pungent odour (due to formation of acrolein) is evolved. Thermal decomposition of
fat gives a complicated mixture of products. It comprises glycerol and esters. In the presence of
dehydrating agents such as potassium hydrogen sulphate, glycerol turns into acrolein.
CH OH CH(OH) CH OH CH =CHCHO 2 HO
KHSO 4
2 2 heat 2 2
3. Test for Proteins:
(i) Biuret test: In this test, a small amount of protein solution is taken and an equal volume of
concentrated alkali solution is added to it. Now, add a drop of 1% CuSO solution, the liquid
4
O
– –
turns bright violet. This test is particular of any compound with peptide linkage – C – NH – as
proteins contain a large number of such groups, they respond to this test by forming a complex with
Cu ions.
2+
(ii) Ninhydrin test: If a protein solution is treated with freshly prepared ninhydrin solution, a blue
precipitate is obtained. This test is given by all proteins except proline and hydroxyproline.
(iii) Millon’s Reagent: To a protein solution, add 2 drops of mercuric nitrate solution and 1 drop of
dilute H SO and then boil it. Cool and add 1 drop of sodium nitrite solution and warm again. A red
4
2
colouration appears.
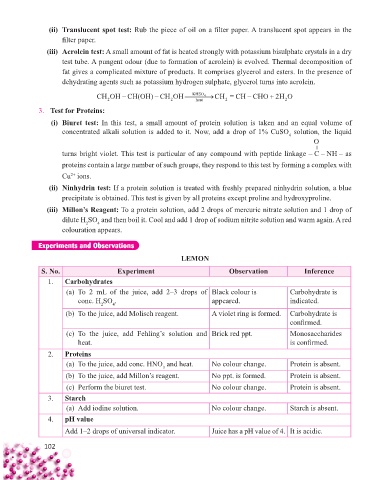
Experiments and Observations
LEMON
S. No. Experiment Observation Inference
1. Carbohydrates
(a) To 2 mL of the juice, add 2–3 drops of Black colour is Carbohydrate is
conc. H SO . appeared. indicated.
2
4
(b) To the juice, add Molisch reagent. A violet ring is formed. Carbohydrate is
confi rmed.
(c) To the juice, add Fehling’s solution and Brick red ppt. Monosaccharides
heat. is confi rmed.
2. Proteins
(a) To the juice, add conc. HNO and heat. No colour change. Protein is absent.
3
(b) To the juice, add Millon’s reagent. No ppt. is formed. Protein is absent.
(c) Perform the biuret test. No colour change. Protein is absent.
3. Starch
(a) Add iodine solution. No colour change. Starch is absent.
4. 4. pH value
pH value
Add 1–2 drops of universal indicator.
Add 1–2 drops of universal indicator. Juice has a pH value of 4. It is acidic.
102