Page 86 - Chemistry - XI
P. 86
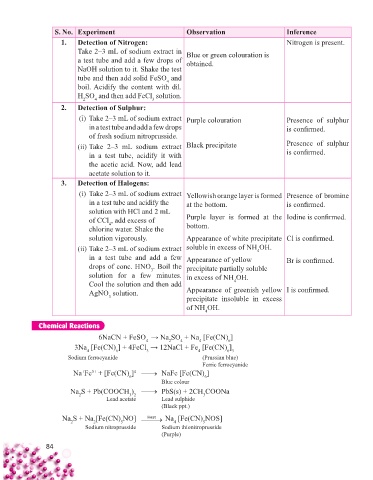
S. No. Experiment Observation Inference
1. Detection of Nitrogen: Nitrogen is present.
Take 2–3 mL of sodium extract in Blue or green colouration is
a test tube and add a few drops of obtained.
NaOH solution to it. Shake the test
tube and then add solid FeSO and
4
boil. Acidify the content with dil.
H SO and then add FeCl solution.
3
4
2
2. Detection of Sulphur:
(i) Take 2–3 mL of sodium extract Purple colouration Presence of sulphur
in a test tube and add a few drops is confi rmed.
of fresh sodium nitroprusside.
(ii) Take 2–3 mL sodium extract Black precipitate Presence of sulphur
in a test tube, acidify it with is confi rmed.
the acetic acid. Now, add lead
acetate solution to it.
3. Detection of Halogens:
(i) Take 2–3 mL of sodium extract Yellowish orange layer is formed Presence of bromine
in a test tube and acidify the at the bottom. is confi rmed.
solution with HCl and 2 mL
of CCl , add excess of Purple layer is formed at the Iodine is confi rmed.
4
chlorine water. Shake the bottom.
solution vigorously. Appearance of white precipitate Cl is confi rmed.
(ii) Take 2–3 mL of sodium extract soluble in excess of NH OH.
4
in a test tube and add a few Appearance of yellow Br is confi rmed.
drops of conc. HNO . Boil the precipitate partially soluble
3
solution for a few minutes. in excess of NH OH.
Cool the solution and then add 4
AgNO solution. Appearance of greenish yellow I is confi rmed.
precipitate insoluble in excess
3
of NH OH.
4
Chemical Reactions
6NaCN + FeSO → Na SO + Na [Fe(CN) ]
4
4
6
2
4
3Na [Fe(CN) ] + 4FeCl → 12NaCl + Fe [Fe(CN) ]
4 6 3 4 6 3
Sodium ferrocyanide (Prussian blue)
Ferric ferrocyanide
Na Fe + [Fe(CN) ] 4 NaFe [Fe(CN) ]
3+
+
6
6
Blue colour
Na S + Pb(COOCH ) PbS(s) + 2CH COONa
3 2
2 Lead acetate Lead sulphide 3
(Black ppt.)
Na S + Na Heat Na [Fe(CN) NOS]
Na S + Na [Fe(CN) NO]
5
2
4
Sodium nitroprusside prusside
2 2 Sodium nitro 5 Sodium thionitroprusside
Sodium thionitroprusside
(Purple)
(Purple)
84