Page 50 - Chemistry - XI
P. 50
Systematic Qualitative
156 Systematic Qualitative
Analysis
Analysis
AND ISOLATION OF DNA
Systematic qualitative analysis is the process of analysing cations and anions present in an inorganic salt
or mixture of salts. We know that salts are formed by the neutralisation of acid and base. All metals form
cations/basic radicals as it comes from base Pb , Al , Mn etc. Acidic radicals/anions are contributed
2+
2+
3+
2
by acid CO , Cl , CH COOetc . Qualitative analysis is carried out through reactions which are easily
3
3
perceptible to our senses, i.e. smell or sight. Such reaction's involve change in colour, evolution of gas, or
formation of precipitate. Qualitative analysis can be divided into dry test and wet test. In general, just by
observing the colour, texture, smell, and other physical properties we can get some important information
about the salt. Tests like heating the salt, fl ame test, borex bead test, charcoal cavity test, sodium carbonate
bead text, blow pipe test are considered as dry test. Some of them are discussed here.
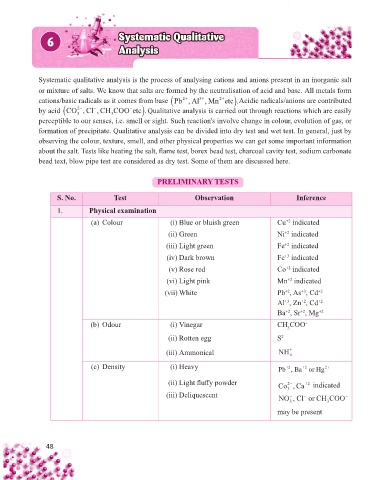
PRELIMINARY TESTS
S. No. Test Observation Inference
1. Physical examination
(a) Colour (i) Blue or bluish green Cu indicated
+2
(ii) Green Ni indicated
+2
(iii) Light green Fe indicated
+2
(iv) Dark brown Fe indicated
+3
(v) Rose red Co indicated
+2
(vi) Light pink Mn indicated
+2
(vii) White Pb , As , Cd +2
+2
+3
Al , Zn , Cd +2
+3
+2
Ba , Sr , Mg +2
+2
+2
(b) Odour (i) Vinegar CH COO –
3
(ii) Rotten egg S 2–
(iii) Ammonical NH + 4
(c) Density (i) Heavy Pb ,Ba or Hg 2+
+2
+2
(ii) Light fl uff y powder Co , Ca indicated
2−
+2
(iii) Deliquescent 3 − − −
NO ,Cl or CHCOO
3 3
may be present
48
48