Page 30 - Chemistry - XI
P. 30
Q4. Which type of buff er is NH Cl + NH OH?
4
4
(a) Acidic (b) Basic (c) Neutral (d) None of these
Q5. Which of the following is true for all acids in aqueous solution?
(a) They conduct electricity . (b) They turn universal indicator red.
(c) They react with all metals to give H gas. (d) They react with ammonium salt to give NH gas.
2
3
Q6. Which of the following is true?
(a) A weak acid does not have a pH =1. (b) A strong acid solution can not have pH = 5.
(c) Weak acid solution will have pH value 4 to 6. (d) Strong acids have pH 1 to 3.
Q7. Which of the following describes correctly the diff erence between HCl and CH COOH of
3
equal concentration?
(a) HCl turns universal indicator orange, but CH COOH turns red.
3
(b) CH COOH reacts more readily with alcohol to form esters than HCl.
3
(c) Electrolysis of HCl liberates H at cathode, but not ethanoic acid.
2
(d) Hydrochloric acid gives a more vigorous reaction with CaCO than ethanoic acid.
3
Q8. Concentration of S will decrease when H S gas is passed in aqueous solution containing HCl
2–
2
due to
(a) common ion eff ect (b) HCl is a weak acid (c) H S is a strong acid (d) all of these
2
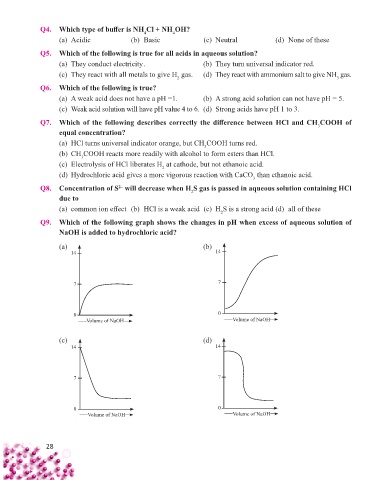
Q9. Which of the following graph shows the changes in pH when excess of aqueous solution of
NaOH is added to hydrochloric acid?
(a) (b)
14 14
7 7
0 0
Volume of NaOH Volume of NaOH
(c) (d)
14 14
7 7
0 0
Volume of NaOH Volume of NaOH
28