Page 28 - Chemistry - XI
P. 28
5. Add 5.0 mL of N/10 HCl from the burette and then add 2 to 3 drops of universal indicator and note
down the pH.
6. Add 7.5 mL of N/10 HCl from the burette and then add 2 to 3 drops of universal indicator and note
down the pH.
7. Add 10 mL of N/10 HCl from the burette and then add 2 to 3 drops of universal indicator and note
down the pH.
Observation Table
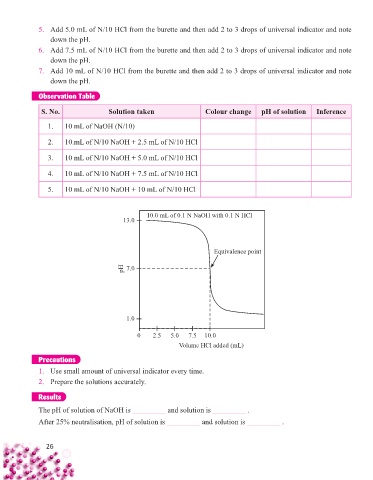
S. No. Solution taken Colour change pH of solution Inference
1. 10 mL of NaOH (N/10)
2. 10.mL of N/10 NaOH + 2.5 mL of N/10 HCl
3. 10 mL of N/10 NaOH + 5.0 mL of N/10 HCl
4. 10 mL of N/10 NaOH + 7.5 mL of N/10 HCl
5. 10 mL of N/10 NaOH + 10 mL of N/10 HCl
10.0 mL of 0.1 N NaOH with 0.1 N HCl
13.0
Equivalence point
pH 7.0
1.0
0 2.5 5.0 7.5 10.0
Volume HCl added (mL)
Precautions
1. Use small amount of universal indicator every time.
2. Prepare the solutions accurately.
Results
The pH of solution of NaOH is _________ and solution is _________ .
After 25% neutralisation, pH of solution is _________ and solution is _________ .
After 25% neutralisation, pH of solution is
26