Page 95 - Biology - XII
P. 95
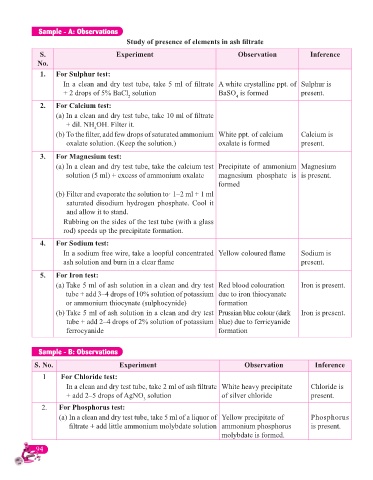
Sample - A: Observations
Study of presence of elements in ash fi ltrate
S. Experiment Observation Inference
No.
1. For Sulphur test:
In a clean and dry test tube, take 5 ml of fi ltrate A white crystalline ppt. of Sulphur is
+ 2 drops of 5% BaCl solution BaSO is formed present.
4
2
2. For Calcium test:
(a) In a clean and dry test tube, take 10 ml of fi ltrate
+ dil. NH OH. Filter it.
4
(b) To the fi lter, add few drops of saturated ammonium White ppt. of calcium Calcium is
oxalate solution. (Keep the solution.) oxalate is formed present.
3. For Magnesium test:
(a) In a clean and dry test tube, take the calcium test Precipitate of ammonium Magnesium
solution (5 ml) + excess of ammonium oxalate magnesium phosphate is is present.
formed
(b) Filter and evaporate the solution to· 1–2 ml + 1 ml
saturated disodium hydrogen phosphate. Cool it
and allow it to stand.
Rubbing on the sides of the test tube (with a glass
rod) speeds up the precipitate formation.
4. For Sodium test:
In a sodium free wire, take a loopful concentrated Yellow coloured fl ame Sodium is
ash solution and burn in a clear fl ame present.
5. For Iron test:
(a) Take 5 ml of ash solution in a clean and dry test Red blood colouration Iron is present.
tube + add 3–4 drops of 10% solution of potassium due to iron thiocyanate
or ammonium thiocynate (sulphocynide) formation
(b) Take 5 ml of ash solution in a clean and dry test Prussian blue colour (dark Iron is present.
tube + add 2–4 drops of 2% solution of potassium blue) due to ferricyanide
ferrocyanide formation
Sample - B: Observations
S. No. Experiment Observation Inference
1 For Chloride test:
In a clean and dry test tube, take 2 ml of ash fi ltrate White heavy precipitate Chloride is
+ add 2–5 drops of AgNO solution of silver chloride present.
3
2. For Phosphorus test:
(a) In a clean and dry test tube, take 5 ml of a liquor of Yellow precipitate of Phosphorus
fi ltrate + add little ammonium molybdate solution ammonium phosphorus is present.
molybdate is formed.
94