Page 94 - Biology - XII
P. 94
5. When the amount of ash contained in various organs of diff erent plants is determined by burning, the
percentage which this ash forms the total dry substance shows a wide range.
Such as, in tobacco leaves it is 17% and in cotton fi bres it forms 1%.
The percentage of ash of separate organs of same plant is diff erent.
6. Leaves give more ash than stem. Ash is nothing, but oxides of elements which are formed during
burning.
Materials Required
Mature tobacco leaves, test tubes, crucible, tripod stand, wire gauze, matchbox, spirit lamp, funnel,
measuring cylinder, pipette, chemicals—conc. HCl, HNO , BaCl , NH OH, ammonium oxalate, potassium
3
2
4
ferrocyanide, potassium thiocyanate, AgNO , ammonium molybdate, uranyl acetate, zinc acetate, acetic
3
acid, etc.
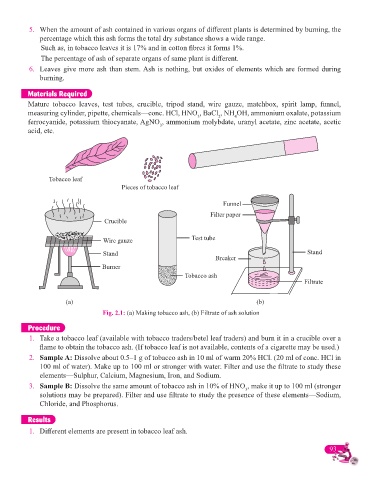
Cigarette
Tobacco leaf
Pieces of tobacco leaf
Funnel
Filter paper
Crucible
Wire gauze Test tube
Stand Stand
Breaker
Burner
Tobacco ash
Filtrate
(a) (b)
Fig. 2.1: (a) Making tobacco ash, (b) Filtrate of ash solution
Procedure
1. Take a tobacco leaf (available with tobacco traders/betel leaf traders) and burn it in a crucible over a
fl ame to obtain the tobacco ash. (If tobacco leaf is not available, contents of a cigarette may be used.)
2. Sample A: Dissolve about 0.5–1 g of tobacco ash in 10 ml of warm 20% HCl. (20 ml of conc. HCl in
100 ml of water). Make up to 100 ml or stronger with water. Filter and use the fi ltrate to study these
elements—Sulphur, Calcium, Magnesium, Iron, and Sodium.
3. Sample B: Dissolve the same amount of tobacco ash in 10% of HNO , make it up to 100 ml (stronger
3
solutions may be prepared). Filter and use fi ltrate to study the presence of these elements—Sodium,
Chloride, and Phosphorus.
Results
1. Diff erent elements are present in tobacco leaf ash.
93