Page 105 - Physics - XI
P. 105
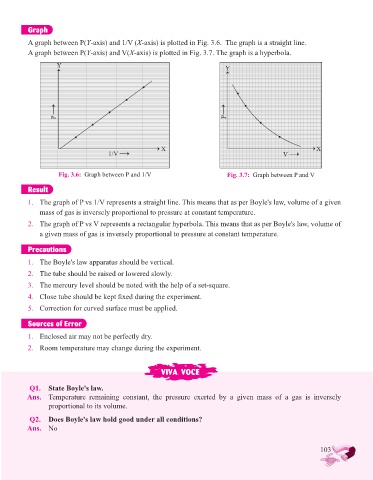
Graph
A graph between P(Y-axis) and 1/V (X-axis) is plotted in Fig. 3.6. The graph is a straight line.
A graph between P(Y-axis) and V(X-axis) is plotted in Fig. 3.7. The graph is a hyperbola.
Y Y
P P
X X
1/V V
Fig. 3.6: Graph between P and 1/V Fig. 3.7: Graph between P and V
Result
1. The graph of P vs 1/V represents a straight line. This means that as per Boyle's law, volume of a given
mass of gas is inversely proportional to pressure at constant temperature.
2. The graph of P vs V represents a rectangular hyperbola. This means that as per Boyle's law, volume of
a given mass of gas is inversely proportional to pressure at constant temperature.
Precautions
1. The Boyle's law apparatus should be vertical.
2. The tube should be raised or lowered slowly.
3. The mercury level should be noted with the help of a set-square.
4. Close tube should be kept fi xed during the experiment.
5. Correction for curved surface must be applied.
Sources of Error
1. Enclosed air may not be perfectly dry.
2. Room temperature may change during the experiment.
VIVA VOCE
Q1. State Boyle's law.
Ans. Temperature remaining constant, the pressure exerted by a given mass of a gas is inversely
proportional to its volume.
Q2. Does Boyle's law hold good under all conditions?
Ans. No
103